
Figure 1. Dry-mass breakdown for large telecommunications spacecraft using nickel-hydrogen batteries (courtesy of British Aerospace)

Whilst some small satellites have design lives of only a year or two, for the majority of larger spacecraft they range from 7 to 15 years (GEO) and 3 to 6 years (LEO) and the trend is for these mission durations to increase. The batteries used must achieve 1000 to 33000 cycles without failure and without any possibility for maintenance. This is considerably in excess of the cycle lives demanded by most terrestrial battery applications and it is the cycle life requirement that has to date confined spacecraft to the use of the well-proven alkaline battery technologies, nickel-cadmium and nickel-hydrogen. However, even when used to 80% of their available capacity, these technologies offer useful mass energy densities of no more than 24 and 36 watt-hours per kilogramme (Wh/kg) respectively at battery level (i.e. taking into account the mass of the battery packaging). The latest lithium-carbon technology promises to revolutionise the world of space batteries in terms of cost/performance factor.
The majority of spacecraft are Earth-orbiting and undergo between 90 and 5500 eclipses per year. The former figure is typical of a geostationary (GEO) telecommunications satellite, whilst the latter is typical of a satellite in low earth orbit (LEO) such as an earth-observation spacecraft. During eclipse, electric power has to be supplied by batteries which are recharged by the solar panels when the spacecraft re-emerges into sunlight. In addition, there are some instances when batteries are called upon to provide peak power in sunlight periods. As can be seen from Figure 1, the existing state-of-the-art batteries (nickel-hydrogen) are heavy, constituting 15% of the dry mass of a typical communications spacecraft and nearly half the mass of the entire payload. Clearly, any mass that could be saved by the use of lighter batteries would allow a corresponding increase in the amount of useful payload equipment. Looked at another way, the use of lighter batteries can be very cost-effective when one remembers that launch costs (GEO) are typically of the order of $50000 per kilogramme. Nickel-hydrogen batteries supplanted the previously-used nickel cadmium batteries because of their mass advantage despite their much higher cost and larger volume.

Figure 1. Dry-mass breakdown for large telecommunications
spacecraft using nickel-hydrogen batteries (courtesy of
British Aerospace)
It has long been realised that there are a number of cell chemistries that should be capable of achieving better than 100 Wh/kg at battery level (based on 100% of capacity). One such class are cells using lithium as the negative electrode and a non-aqueous electrolyte. There is a large range of candidate positive electrode materials. Although work on these types of cells has been continuous since the early seventies, successful commercial application of rechargeable lithium cells has been extremely limited because of the poor cycle life that has been achievable (at most a few hundred cycles) and to some extent because of concerns over safety. (In contrast lithium primary cells have found extensive application, though safety concerns limit their domestic use in large capacity versions).
The poor cycle life was due to the difficulty of re-plating lithium onto the negative electrode during recharge. A significant proportion of the lithium re-plated during each cycle is not adequately integrated into the electrode and can no longer be discharged. Hence the loss in capacity. Again it has long been realised that this might be overcome by switching from the use of metallic lithium as the negative electrode to another material which could 'dissolve' lithium in a solid structure in which the lithium atoms are nevertheless mobile (one of the authors worked on this approach in the late seventies). The problem was that the mass of lithium which could be reversibly dissolved into the materials that were tried was too small compared to the mass of the electrode, negating most of the energy-density advantage one was trying to obtain.
A major breakthrough was achieved by Sony when, in 1991, it introduced cells in which the lithium metal was replaced by a layered-structure carbon electrode into which lithium ions can pass reversibly and in large quantity (roughly one lithium atom per six carbon atoms). This idea had been tried in the past without success, but the breakthrough was in finding the right form of carbon and the necessary pre-treatments for it to function reversibly. The use of carbon in place of lithium metal still does not come without penalties. It results in a reduction of about 0.3 volts in cell potential and it adds to the mass of the negative electrode. Fortunately, developments in positive electrode materials had shown how to compensate for this voltage disadvantage and, perhaps still more importantly, opened the way to simpler cell manufacture.
Unlike the negative electrode, positive electrodes have nearly always been 'solid solution electrodes' in which lithium ions are free to move within a layer structure, usually a transition metal oxide or sulphide. The content of lithium ions can be varied over a wide range of composition because the lithium ion charge can be balanced by the variable charge of the transition metal ions. Earlier lithium cells were made in the charged state, i.e. by combining lithium-metal negative electrodes with positive electrodes containing no lithium. As the cell was discharged, the voltage associated with the positive electrode fell as lithium ions were introduced.
The breakthrough in positive electrodes came from a UK Atomic Energy Authority (now AEA Technology) programme, as part of which J. Goodenough at Oxford University devised a new class of positive solid solution electrode materials that were already lithium-containing as synthesised. Combined with the new carbon positive electrodes, this brought two important advantages. Firstly, it enabled cells to be assembled in the discharged state so that it was no longer necessary to handle metallic lithium (or the still very air- and water-sensitive lithium-carbon compound) during manufacture. This greatly simplified the manufacturing process. Secondly, when lithium was removed from the positive electrodes during charge, the voltage associated with the electrode increased, largely compensating the voltage penalty associated with the negative.
Any cells based on lithium-carbon negative electrodes and solid solution positive electrodes are referred to as lithium- carbon or lithium-ion cells. The latter name refers to the fact that the cell can be regarded as a concentration cell in which lithium remains in the form of ions and the voltage is due to the difference in chemical activity between the positive and negative electrodes. The operation of such a cell is shown diagrammatically in Figure 2. They are now under vigorous development by most major battery manufacturers around the world and especially in Japan for use in portable electrical equipment such as computers, power tools and for electric vehicles. In fact, lithium-ion cells are not restricted to graphite as the anode host to the lithium ions, and alternatives are also under development.

Figure 2. Schematic of the lithium-carbon/ion cell's
operation
(courtesy of AEA Technology)
A further development, begun in the early eighties, has been to replace the liquid organic electrolyte with a lithium-ion- conducting polymeric electrolyte. This opens up a way to fabricate all solid-state batteries and to further save on battery mass by obviating the need for the battery container to provide mechanical strength as well as hermeticity. Thin- card geometries are attractive with this technology and it also makes more feasible the fabrication of bipolar batteries where a number of couples are stacked in series within the same package. The down side of this approach is an increase in electrolyte resistance, especially at lower temperatures, which reduces the cellOs rate capability compared to a liquid- electrolyte cell of the same geometry.
Typical lithium-carbon cell (deep) cycle lives currently reported are still quite modest at around 1000 to 2000 cycles. This is nevertheless sufficient to be worthy of serious consideration for GEO spacecraft as well as for limited-life small satellites. These are also the space applications which are growing the most rapidly and account for the vast majority of spacecraft to be launched in 1997. In short, the opportunity to halve the battery mass on these spacecraft at a cost which should not exceed that of the current state of the art is too attractive to pass over. Figure 3 compares the projected masses and volumes of lithium-carbon batteries with those of current technologies. It can be seen that the lithium-carbon battery is projected to be very compact, especially compared to current-generation independent pressure vessel (IPV) nickel-hydrogen batteries.
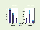
Figure 3. Mass and volume of lithium-carbon batteries compared
to current technologies data are for IPV Ni-H2 and
cylindrical cell Li-C
(courtesy of SAFT)
Another advantage is that these cells have a voltage 2.5 to 3 times that of the alkaline cell technology, thus reducing the number of cells required per battery by the same ratio. Yet another valuable feature for certain space applications is their 'magnetic cleanliness'. The electrode substrate employed in nickel-cadmium and nickel-hydrogen batteries is of course nickel, a (ferro-)magnetic metal. This excludes them from use aboard scientific spacecraft carrying experiments involving sensitive magnetometers. There have been a series of ESA spacecraft in this category: Heos, Geos, Giotto and, most recently, Cluster. All have had to use silver-cadmium batteries, which although having quite a good energy density (around 70 Wh/kg at battery level), have poor storage and cycle life performances. The requirements of Cluster are in fact quite close to the limits of this technology. Lithium- carbon cells need not contain ferromagnetic materials and have already demonstrated superior performance to silver-cadmium. Whilst this type of application is not commercial, use of lithium-carbon should allow longer missions and increase the scientific return.
The excellent storage life expected for lithium-carbon might also make it attractive for powering certain deep-space probes and planetary landers where there is a requirement for a limited number of recharges. The current alternative, silver- zinc, which has been chosen for the US Mars Pathfinder mission, suffers from the disadvantage of having a limited storage life once it has had its electrolyte added. To get around this limitation, cells with remote activation have been designed in the past, but at considerable penalty in cost, mass and complexity. Lithium-carbon would not require such measures. The technology would be particularly valuable if it could be designed for operation at lower temperatures, down to -20°C or below.
As an important part of its Technological Research Programme (TRP) and in collaboration with CNES, ESA started a development of lithium-carbon (organic electrolyte) cells for space applications at SAFT (F) in 1994. The small (7 Ah) prototype cells were subsequently evaluated in the European Space Battery Test Centre at ESTEC (NL) and gave encouraging results. The second phase of the development is currently in progress. Here the target is to produce qualifiable cells in the 40 to 100 Ah range, suitable for future telecommunications spacecraft. These cells are also baselined for flight aboard Stentor, a technology-demonstration spacecraft (Fig. 4) which is due to be launched in 2000. The development is building on the manufacturer's much larger programme for the future electric-vehicle market, which will require cells of similar capacity. The development and qualification of a complete battery system for large geostationary spacecraft features prominently in the new ESA Technology Plan for 1997-9.

Figure 4. The Stentor technology-demonstration spacecraft
(courtesy of CNES)
As mentioned earlier, another promising area of application is the 'small-sat' market. Here, battery capacities in the region of 2 to 5 Ah are sufficient, a size already available commercially from certain Japanese manufacturers for portable electronic equipment. In this field, low cost is all-important so an attractive approach is to use commercial cells rather than the traditional approach of using cells built especially for space applications in small numbers and at high cost. The STRV 1 C and D spacecraft, due to be launched together in 1998, will use battery modules containing Sony commercial cells (Fig. 5), and ESA is embarking on a General Support Technology Programme (GSTP-2) at AEA Technology (UK) aimed at scaling up this modular concept to cover small-to-medium size applications and to meet the more stringent reliability and lifetime requirements that go with them.

Figure 5. A six-cell battery module for STRV
(courtesy of
AEA Technology/BNSC)
ESA thus regards these two development programmes as complimentary. Together, they cover most space applications not requiring more than, say 5000 charge-discharge cycles, a modest increase over the current capability of this technology and one which stands a good chance of being reached within the frame of the parallel terrestrial developments.
To cover deep-space needs, it is intended in the future to investigate improving cell performance at lower temperatures. Whether the cycle life can be improved sufficiently to meet long LEO mission requirements is as yet unknown. Laboratory evaluation of the lifetime of current commercial cells as a function of depth of discharge, temperature and charge control are planned at ESTEC in order to understand which factors most influence cycle life.
Within its ASTP-4 programme, ESA also has an ongoing activity at Danionics (DK) for the development of a lithium secondary battery in which the liquid electrolyte is replaced by a lithium-ion conducting polymer. For space applications one has to trade off the advantage of ruggedness against the lower expected rate capability of this configuration. There are, however, reasons to hope that a polymer electrolyte may allow a greater cycle life to be achieved which would be an important advantage. A prototype polymer electrolyte battery from this programme is due for delivery to ESTEC later this year for life-testing.
Life-cycling provides information not only on the 'graceful' degradation in performance that inevitably accompanies cell ageing, but also on possible premature failure modes. Whilst most of these can be eliminated by good manufacturing control, it is sometimes impossible to reduce the risk to such low levels that it can be ignored. Part of any space-battery design is a failure mode and effects analysis (FMECA), which has to include all conceivable ways in which a battery and its cells and other components may fail and to assess the impact of each of these failure modes on the battery's subsequent performance. To meet reliability requirements, a battery design may have to include appropriate means for minimising the impact of a failed cell. In the case of nickel-hydrogen, for example, it is usually necessary to provide means for bypassing a cell that fails in open circuit. This sort of information for lithium secondary cells is only beginning to become available, yet is essential for optimising the design of a space battery.
Charge control of lithium-carbon batteries is an important aspect of the technology, and initial conceptual and breadboard studies at ETCA (B) (part of the Agency's MSTP programme) are nearing completion. Charge control is important because of a fundamental difference between lithium secondary batteries and the presently used alkaline systems. Both nickel-cadmium and nickel-hydrogen cells are capable of accepting significant overcharge without damage. Overcharging results in generation of oxygen at the positive electrode, but the design of the cells allows this oxygen to recombine chemically at the negative electrodes, preventing an excessive buildup of pressure that would otherwise occur in a sealed cell. There is therefore no net change in the cell as a result of overcharge, except for the heat generation which accompanies the recombination reaction.
Whilst overcharging a battery wastes energy, a limited amount of overcharge is beneficial because it ensures that all of the cells, connected in series within the battery, reach full charge at the end of the charging period. There is always the risk that some cells will have or will develop a greater self- discharge or leakage current than others so that without overcharge and over a large number of cycles these cells can 'run down', eventually reducing the useful capacity of the battery to levels insufficient to meet the needs of the payload during eclipse. Lithium secondary cells in contrast do not possess such a 'safe' overcharge reaction mechanism. Small amounts of overcharge can irreversibly damage a cell's subsequent performance and excessive overcharging can lead to the buildup of internal pressure leading to bursting. It is therefore not certain that it will be possible to maintain all cells in a battery at the same state of charge without additional means to adjust the state of charge of individual cells.
The high round trip efficiency (the useful energy recoverable on discharge divided by the energy required to charge) of lithium carbon (typically >90%) is another advantage compared to the currently used batteries which have efficiencies nearer to 80%. This results in a halving of the heat generation in the battery. Together with the somewhat higher maximum temperature of operation (probably about 40°C unless the cycle life turns out to be unduly compromised), this means that the thermal design of lithium- carbon batteries will be less critical and there should be additional mass savings to be gained in battery thermal- control elements such as battery radiator plates.
Battery thermal control is often critical because the batteries are usually one of the greatest sources of heat dissipation onboard a spacecraft, but also have one of the smallest allowable temperature ranges of operation. Battery thermal control would therefore be further simplified if the temperature range of operation of lithium batteries turns out to be larger than for nickel-cadmium and nickel-hydrogen (typically -5 to +25°C). At the moment, however, it is not sure what the practical lower temperature limit for lithium-carbon batteries is going to be.
It is an exciting time in the space battery field. Lithium- carbon promises to revolutionise space batteries, providing a much bigger step up in performance than that which was achieved in the switch from nickel-cadmium to nickel-hydrogen. GEO spacecraft using this technology will have a significant competitive edge over those using nickel-hydrogen. It should also provide a considerable cost/performance advantage for small satellites, as well as improve mission capability for certain scientific spacecraft. In the interests of maintaining European competitiveness, it is essential that the greatest possible effort be made to qualify this technology for space as quickly as possible.



 ESA Bulletin Nr. 90.
ESA Bulletin Nr. 90.