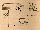
Fig. 1. Layout of the TEM 06-5 electrofusion module.

In plants, somatic hybridisation and the exchange or recombination of organelles between incompatible species can be achieved by fusion of plant cell protoplasts. Early attempts to induce protoplast fusion successfully employed high pH and calcium, polyethylene glycol or dextran. Another approach is the fusion of protoplasts by the reversible electric breakdown of their plasma membranes.¹ With this method, protoplasts are first brought into close membrane contact by a weak alternating electrical field ('positive electrophoresis ), followed by a field pulse of high intensity and short duration to induce local membrane reorganisation at the contact areas of the two spheres. This approach has been shown to be an efficient alternative to conventional methods as it avoids chemical interference of the fusogens with cell properties. Furthermore, electrofusion is the method of choice if selected hybrids are to be formed.
In spite of the considerable potential offered by this field pulse technique, optimum yields of hybrids are obtained only if the electrically-aligned cells do not change their positions for a certain time after pulse application. Only then can persistent membrane continuities develop. Under terrestrial conditions, however, gravitational and convectional forces severely interfere. For example, cell nuclei or organelles, such as chloroplasts or amyloplasts, are not centred and thus cell rotation occurs. It is therefore imperative to maintain higher field strengths for longer in order to keep the fusion partners aligned. In addition to suboptimal fusion yields, this can cause a decrease in hybrid viability.
These limitations become even more stringent when protoplasts of different specific density are to be fused, such as cells with or without vacuoles. Such types of protoplasts are present in differentiated leaf (vacuolated) and in meristematic tissues (largely free of vacuoles). They can also be formed experimentally by evacuolisation of, for example, ordinary leaf cell protoplasts.
Owing to the detrimental effects of differential sedimentation velocity of protoplasts of different specific density, it was our hypothesis that electrofusion under microgravity should considerably increase yields of viable fusion products.
There is also evidence from experiments with cell cultures and animals that changes in gravitation should interfere with cell metabolism. For example, it has been shown that, under µg, unicellular organisms (Paramecium²) exhibit reduced mobility but increased rates of cell division. It was suggested therefore that a surplus of energy resulting from decreased motional activities is available for other cellular processes. It thus appeared interesting to screen for possible metabolic responses to changes in gravitational forces.
Here we report on the effects of µg on plant cell protoplasts, yield and viability of fusion products after electrofusion, and changes in pool sizes of adenylates (ATP, ADP) and of fructose 2,6-bisphosphate, a metabolite regulator of glycolysis. The experiments were performed as part of the TEXUS sounding rocket programme (TEXUS 17, 21, 25, 30). Possible changes in adenylate ratios were also tested on a slowly rotating clinostat,³ but the results were inconsistent.
Plant material
The preparation of
vacuolated (P+) and evacuolated (P-) mesophyll protoplasts of
Nicotiana tabacum (cv. Samsun) or Avena sativa,
determination of protoplast and fusion product viability, and
enrichment of heterospecific 1:1 fusion products was as detailed
in ref. 4.
Hardware design for remotely-controlled electrofusion
and data transmission
The module used for the
experiment on TEXUS 17 & 21 consisted of the fusion chamber, two
storage units for vacuolated and evacuolated protoplasts, a
reservoir for the fusion medium, a peristaltic pump and
facilities for visual control of the fusion chamber s inter-
electrode space. 4 The different containers were
connected by silicon tubing (1 mm inner diameter). In order to
compensate for changes in volume during resuspension of the
protoplasts and filling of the fusion chamber, all storage units
(containers for protoplasts and the fusion medium) were closed
at one end by flexible membranes (Freudenberg, Weinheim,
Germany). This design was modified for TEXUS 25 as shown in Fig.1.
Resuspension of protoplasts by a stirring bar resulted in
highly homogeneous protoplast mixing and chamber filling.
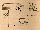
Fig. 1. Layout of the TEM 06-5 electrofusion module.
In order to introduce significant numbers of protoplasts into the fusion process, a meander-shaped fusion chamber was constructed (Fig. 1). The stainless steel electrodes were fabricated by spark-erosion (Witte, Barskamp, Germany). The 1.2 mm electrode separation, 8 mm gap height and 520 mm total length of the inter-electrode space allowed about 5 ml of protoplast suspension to be exposed to the required electric field. For visual control of the inter-electrode space, the electrode housing was made of plexiglass. The fusion chamber was mounted on a movable platform, where a step motor could position it in the light path of the microscope optics (perpendicular to the chamber surface; total magnification 100-200x). The complete experimental unit (except the optics) was mounted on a separate platform which could be introduced into the module through a lateral window. This allowed the samples to be inserted into the assembled payload 2 hr before launch. Thermal control of the cell containers was achieved by Peltier elements. This was necessary in order to maintain the protoplast suspensions at 6-8°C until about 10 min before launch and at 20°C during the electrofusion experiment.
Conditions for electrofusion of vacuolated and
evacuolated tobacco mesophyll protoplasts (TEXUS 17 & 21)
The peristaltic pump was activated about 80 s after
launch and both types of protoplasts (0.4 ml each) were
resuspended by dilution in their storage units to 8 ml with
fusion medium (0.5 M sorbitol). By changing the pumping
direction, the protoplasts were drawn from their respective
chambers under continuous mixing in a ratio of 1:1. Times for
mixing and filling were 15-20 s each, at 20°C. After
completing the filling procedure, protoplasts were collected by
a weak alternating field (2 MHz, 160 V/cm peak to peak) under
visual control and as soon as short protoplast chains were
visible the fusion pulse was applied (0.9 kV/cm, 50 µs).
Some of the sample processing was automatic but critical steps
(pump activation, flow direction and pulse application) could be
directed by telecommand.
Evaluation of fusion yield and physiological integrity
of fusion products (TEXUS 17)
Within 1 hr of pulse
application (similarly for the 1 g reference), the
protoplast suspension was washed out of the fusion chamber by
about 50 ml of 0.5 M sorbitol, containing 1 mM CaCl 2
and 5 mM Mes-KOH (pH 6). Pre-separation of particle populations
of different densities was by sedimentation for 1 hr under 1
g at 6°C. This procedure was repeated with the
remaining supernatant. For enrichment of heterospecific 1:1
fusion products, the pellets obtained by these sedimentation
steps were combined with the pellet resulting from centrifuging
the second supernatant (10 min at about 50 g) and layered
on top of a pre-formed Percoll gradient. 5 The yield
of heterospecific fusion products (vacuolated x evacuolated
protoplast) was determined microscopically by counting aliquots
of the inital washout on a hemocytometer (Fuchs-Rosenthal).
Physiological integrity of fused protoplasts was tested by their
individual ability to evolve oxygen photosynthetically. The
assay, which employed aerotactic bacteria (Pseudomonas
aeruginosa) is described in detail in ref. 6.
Culture of protoplast/fusion products and regeneration
of plants (TEXUS 21 & 25)
For sterile culture,
pulse-treated protoplasts were washed once with 0.5 M mannitol
buffer, containing 1 mM CaCl2, 5 mM 4-
morpholinoethanesulfonic acid (MES, pH 6.0) and resuspended in
modified 12a medium which contained macro- & micro-elements,
vitamins, amino acids, 4.7 mg/l p-chlorophenoxyacetic acid and
1 mg/l kinetin, all supplemented with 3% (w/v) sucrose and 9%
(w/v) mannitol. Aliquots of the pulse-treated suspension were
embedded in 1% (w/v) low gelling temperature agarose (Sea Plaque,
FMC Bio-Products, Rockland, USA). Droplets (100 µl each) of
the agarose protoplast suspension were transferred into 6 cm-
diameter petri dishes and incubated in the dark at 22°C in
the presence of feeder cells (tobacco mesophyll protoplasts or
carrot suspension culture cells; 50 000 & 100 000/ml). Tobacco
mesophyll protoplast feeder cells were diluted every 10 days with
1 ml of osmoticum-free culture medium. Carrot suspension culture
cells were diluted at intervals of 4 days by replacing 1 ml of
suspension with 1 ml of culture medium of decreasing osmolarity.
After 6 weeks, the agarose lenses free of feeder cells were
transferred on to agar medium. Seven days later, microcalli were
dispersed on the agar. After another 2 weeks, the calli were
transferred on to shoot regeneration medium. Developing shoots
were cut off and rooted. Regenerants were tested for patterns of
selected enzymes (esterases, glutamate oxalacetate transaminase)
by polyacrylamide gel electrophoresis.
Assay of pool sizes of metabolites (TEXUS 25 &
30)
For the analysis of changes in pool sizes of
metabolites, a module was constructed (MBB/ERNO) comprising 31
sample containers of adjustable volumes (0.2-1 ml, pressure-
driven piston) and a pressure-driven injection device for a
quenching agent (Fig. 2).
Fig. 2. Layout of the unit used for metabolic quenching of protoplast suspensions.
Oat mesophyll protoplasts (0.2 ml, 0.2x106)7 were loaded into the sample cavities (set to 0.2 ml) about 2 hr before launch and kept at 6- 8°C. The sample temperature was increased to 20°C and the whole device activated 30 s before launch by injection of 0.8 ml ethanol into the first sample; the remaining samples were then mixed with the quenching agent at 14 s intervals. Ethanol was used because it allowed for the assay of both acid-stable (ATP, ADP) and acid-labile (fructose 2,6-bisphosphate) compounds from the same extract. Assay of adenylates was by luminometry;8 fructose 2,6-bisphosphate was determined as described in ref. 9.
Electrofusion of a mixture of vacuolated and
evacuolated tobacco mesophyll protoplasts under terrestrial
gravitation (1 g)
The experimental set-up as
described above was used for control experiments under
terrestrial gravitation. In order to obtain optimum fusion rates,
the fusion chamber was filled under different spatial
orientations. The best results were obtained with the meander s
long side perpendicular to the gravitational field (see Fig. 1).
Independent of the chamber orientation, the mixing and filling
procedure resulted in a highly homogeneous distribution of both
vacuolated and evacuolated protoplasts over the total length of
the inter-electrode space. Immediately after filling, however,
a rapid separation of both particle populations started, i.e.
while the vacuolated protoplasts remained suspended the
evacuolated ones sedimented. This led to a highly inhomogeneous
distribution of both types of protoplasts within
seconds,4 which was only slightly retarded by the
immediate application of the alternating electric field (positive
dielectrophoresis). Thus, primarily homospecific pair and chain
formation occurred (P(+) x P(+); P(-) x P(-)). As a consequence,
electric pulse-induced fusion created only small numbers of P(+)
x P(-) fusion products (0.7-1.0 % of the total protoplast
population).
Electrofusion under microgravity
Remotely-controlled mixing and filling of the fusion chamber
under µg caused a homogeneous distribution of both
protoplast preparations that was completely stable when the
filling step ended. The only visible movement of the protoplasts
relative to each other occurred when the collecting AC field was
applied. After a 20 s AC field, protoplast pairs started to grow
into chains of several protoplasts, so the alternating field was
switched off. Fusion was initiated by setting a square pulse (0.9
kV/cm, 50 µs) and, again, no particle movement was visible.
This is in significant contrast to fusion under 1 g; here,
sedimentation and convectional forces induce relative movement
of the fusion partners, which eventually leads to a breakdown of
newly-formed membrane continuities. Thus, a post-fusion weak
alternating field typically has to be applied in order to prevent
a separation of the fusion partners. This, however, affects the
viability of fusion products (unpublished observation). Our
experimental data show that this is not necessary under
weightlessness.
Microscope analysis of the exposed protoplast suspension after retrieval (less than 1 hr after fusion) showed a significantly increased portion of fusion products, of both homospecific and heterospecific nature. Evaluation of about 1000 protoplasts (vacuolated and evacuolated) yielded about 120 clearly distinguishable 1:1 fusion products, i.e. about 12% of all cells submitted to the fusion procedure. This is about 10-15 times more than fusion under terrestrial conditions with the same hardware; in real numbers, about 0.5x106 heterospecific 1:1 fusion products out of 4x106 protoplasts introduced into the fusion chamber.
Similarly, the yield of homofusion and multifusion products (fusion products formed from either vacuolated or evacuolated protoplasts) also increased, although to a lesser extent. In this case, the yield increase should not be a matter of missing differential sedimentation velocity, as, owing to the isolation procedure (purification on a density gradient), there should be no larger difference in specific density within such a protoplast population. Instead, we believe the lack of convectional movement increased the yield. Under terrestrial gravity, a weak alternating electric field has to be applied after pulse application in order to prevent membrane bridges from breaking down. In a solution without any electrical conductivity this would not cause thermal problems. There is, however, always some leakage of solutes from decomposing protoplasts, which increases conductivity. Thus some current will pass the protoplast suspension and cause local increases in temperature. This causes convectional movement and, finally, a decreased number of fused cells.
As far as fusion products from protoplasts with comparable specific density (P(+) x P(+); P(-) x P(-)) could still be identified beyond doubt (about 2-4 hr after µg fusion), the yield with respect to the total number of vacuolated protoplasts was about twice that under terrestrial conditions (10.5% instead of 4.5%). This is, however, an underestimate. Owing to the more or less rapid reorganisation of a vacuolated fusion product from tobacco mesophyll protoplasts, we were possibly able to identify only a fraction of the totally formed fusion products. Under terrestrial conditions only up to 50% of the fusion products recognisable within minutes after electrofusion can be identified by recounting 2 hr later (storage at 4°C). As the µg-exposed samples were kept under comparable conditions, the real increase in yield could be considerably higher. Such an evaluation is not a problem with fusion products formed from vacuolated x evacuolated protoplasts. Here, the viscosity of the cytoplasm of the evacuolated partner is so high that complete mixing takes up to 2 days.
Centrifuging of the pulsed protoplast suspension on a pre- formed sigmoidal Percoll gradient yielded a fraction enriched with up to 27% heterospecific 1:1 fusion products. Fusion products from this fraction were assayed for physiological integrity by qualifying their indiviual ability for photosynthetic oxygen evolution. This test system, employing aerotactic bacteria (Pseudomonas aeruginosa), indicated that nearly all fusion products (>90%) obtained by electrofusion under µg were viable according to this standard. This is significantly more compared to terrestrial conditions (about 50-60%).
Regeneration of tobacco plants from pulsed suspensions
of vacuolated and evacuolated mesophyll protoplasts
Finally, with the fusion experiment during the TEXUS
21 & 25 missions, we showed that the whole experimental procedure
can also be performed under sterile conditions. As a result, we
obtained hybrid plants resulting from the fusion of vacuolated
with evacuolated tobacco mesophyll protoplasts. These regenerates
expressed morphological characteristics that were intermediate
to those of the parental plants, Nicotiana tabacum (cv.
Samsun; evacuolated protoplasts) and Nicotiana
rustica.10
Pool sizes of metabolites during transients in
gravitational forces (TEXUS 25 & 30)
The ratio of
ATP/ADP and the amount of fructose 2,6-bisphosphate in extracts
of oat mesophyll protoplasts were clearly affected by the
decrease in gravitational forces to <10-4g. The
ratios of ATP/ADP started to fluctuate and the pool size of
fructose 2,6-bisphosphate increased upon transition to µg.
In control experiments we could show that under the conditions
described respiration of protoplast suspensions was rather steady
(oxygen electrode). Thus, the alterations shown should not be
dependent upon changes in oxygen availability. This is supported
by the reference experiment (1 g), which did not result
in comparable variations of pool sizes or adenylate ratios.
From the literature and our own experiments,11 we know
that an increase in fructose 2,6-bisphosphate limits
sucroneogenesis and indicates an increase in glycolytic activity.
This possibly indicates a higher demand for metabolic energy -
increased rates of active transport due to decreased rates of
diffusion under µg because of larger unstirred layers (no
convection?). These findings do not warrant further comment
because they result from a very limited number of experiments.
We have shown that electrofusion under weightlessness can be used to increase significantly the yield of viable fusion products from parental protoplasts with considerable differences in specific density as detrimental effects caused by particle sedimentation and convectional forces are excluded. Recovery of fusion products under terrestrial conditions allows the regeneration and propagation of hybrid plants. Our preliminary experiments on metabolic responses during g-transients suggest adaptions of the energy metabolism.
The authors are indebted to the Bundesministerium für Forschung und Technologie for financial support and to both DARA and MBB/ERNO for logistical support.



 SP1206
SP1206