Accept all cookies Accept only essential cookies See our Cookie Notice

About ESA
The European Space Agency (ESA) is Europe’s gateway to space. Its mission is to shape the development of Europe’s space capability and ensure that investment in space continues to deliver benefits to the citizens of Europe and the world.
Highlights
ESA - United space in Europe
This is ESA ESA facts Member States & Cooperating States Funding Director General Top management For Member State Delegations European vision European Space Policy ESA & EU Space Councils Responsibility & Sustainability Annual Report Calendar of meetings Corporate newsEstablishments & sites
ESA Headquarters ESA ESTEC ESA ESOC ESA ESRIN ESA EAC ESA ESAC Europe's Spaceport ESA ESEC ESA ECSAT Brussels Office Washington OfficeWorking with ESA
Business with ESA ESA Commercialisation Gateway Law at ESA Careers Cyber resilience at ESA IT at ESA Newsroom Partnerships Merchandising Licence Education Open Space Innovation Platform Integrity and Reporting Administrative Tribunal Health and SafetyMore about ESA
History ESA Historical Archives Exhibitions Publications Art & Culture ESA Merchandise Kids Diversity ESA Brand Centre ESA ChampionsLatest
Space in Member States
Find out more about space activities in our 23 Member States, and understand how ESA works together with their national agencies, institutions and organisations.
Science & Exploration
Exploring our Solar System and unlocking the secrets of the Universe
Go to topicAstronauts
Missions
Juice Euclid Webb Solar Orbiter BepiColombo Gaia ExoMars Cheops Exoplanet missions More missionsActivities
International Space Station Orion service module Gateway Concordia Caves & Pangaea BenefitsLatest
Space Safety
Protecting life and infrastructure on Earth and in orbit
Go to topicAsteroids
Asteroids and Planetary Defence Asteroid danger explained Flyeye telescope: asteroid detection Hera mission: asteroid deflection Near-Earth Object Coordination CentreSpace junk
About space debris Space debris by the numbers Space Environment Report In space refuelling, refurbishing and removingSafety from space
Clean Space ecodesign Zero Debris Technologies Space for Earth Supporting Sustainable DevelopmentLatest
Applications
Using space to benefit citizens and meet future challenges on Earth
Go to topicObserving the Earth
Observing the Earth Future EO Copernicus Meteorology Space for our climate Satellite missionsCommercialisation
ESA Commercialisation Gateway Open Space Innovation Platform Business Incubation ESA Space SolutionsEnabling & Support
Making space accessible and developing the technologies for the future
Go to topicBuilding missions
Space Engineering and Technology Test centre Laboratories Concurrent Design Facility Preparing for the future Shaping the Future Discovery and Preparation Advanced Concepts TeamSpace transportation
Space Transportation Ariane Vega Space Rider Future space transportation Boost! Europe's Spaceport Launches from the European Spaceport from 2012Latest

Iron burning
Thank you for liking
You have already liked this page, you can only like it once!
Iron dust burning. Everything burns. Given the right environment, all matter can burn by adding oxygen, but finding the right mix and generating enough heat makes some materials combust more easily than others. Researchers interested in knowing more about a type of fire called discrete burning used ESA’s microgravity experiment facilities to investigate.
Burning iron dust in experiments on zero-g aircraft and rocket flights allowed for the iron particles to float and ignite discreetly. High-speed cameras captured the spectacle and allowed the researchers to better understand the phenomenon, resulting in computer models that showed the ideal conditions to burn the fuel on Earth.
With the new understanding made possible from microgravity research it became possible to build efficient and practical iron-burning furnaces.
The advantage of burning iron is down to chemistry. Essentially, burning fuel is the process of transforming a material by adding oxygen atoms. his is why carbon-based fuel produces the greenhouse gas carbon dioxide when two oxygen atoms are added to the carbon-based fuel such as wood, coal or oil. With iron, the leftover product after combustion is iron oxide, more commonly known as rust. No carbon dioxide is produced, and the rusty iron can be easily collected as it doesn’t form a gas – burning iron emits no noxious gases at all.
Iron rust can even be processed to remove the oxygen and return it as iron using hydrogen. By using electricity from sustainable sources, iron as a fuel can become a circular, endlessly recyclable energy storage.
A demonstration plant is already up and running in Budel, near Eindhoven, The Netherlands, using iron as its fuel source this generator can produce 1 MW of steam in a unit that stands in a warehouse. Scaled up such an iron power plant could produce much more energy.
Multiple start-ups are already pursuing this carbon-free fuel, to power factories and industrial processes.
-
CREDIT
Iron+ -
LICENCE
ESA Standard Licence

Iron fuel demonstration plant

Iron furnace demonstration plant

Iron’s in the fire
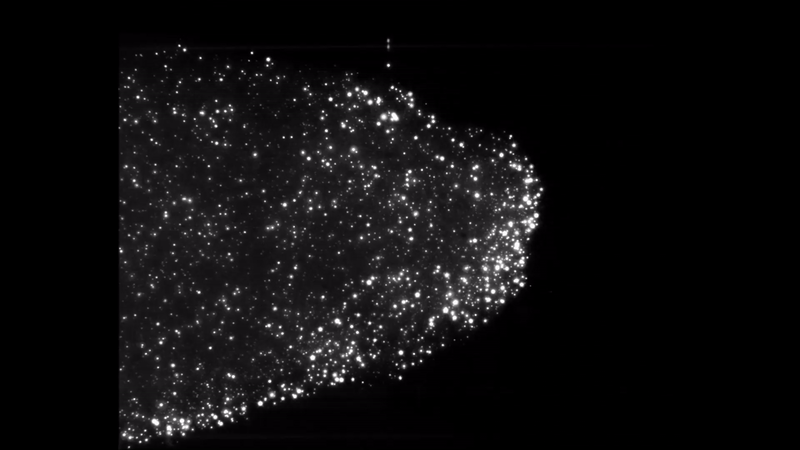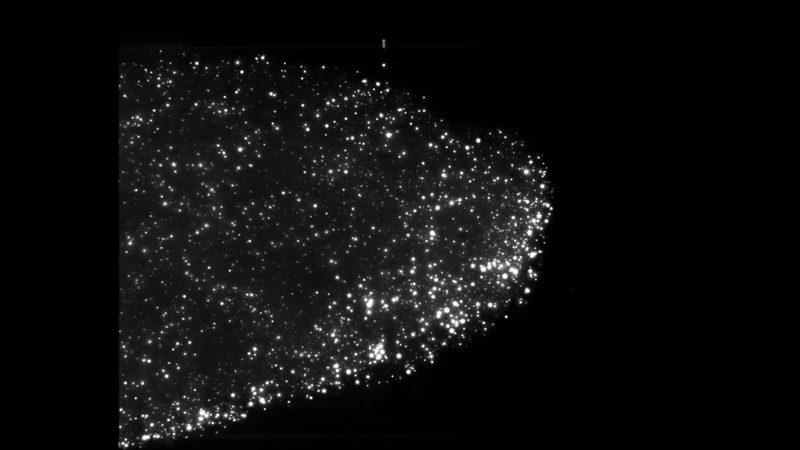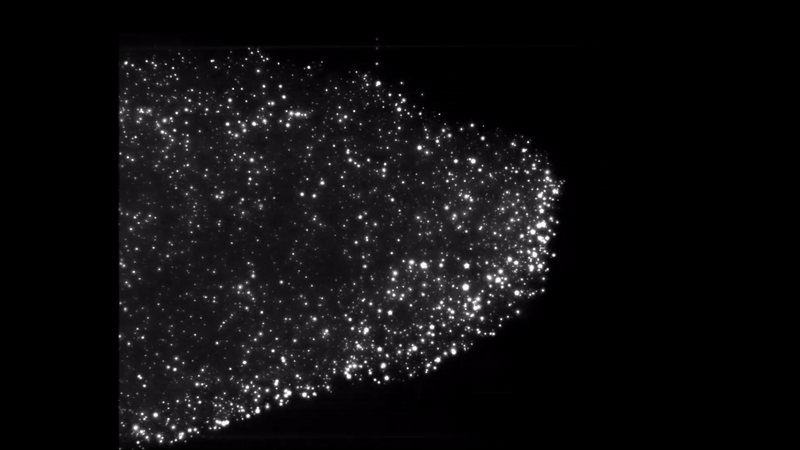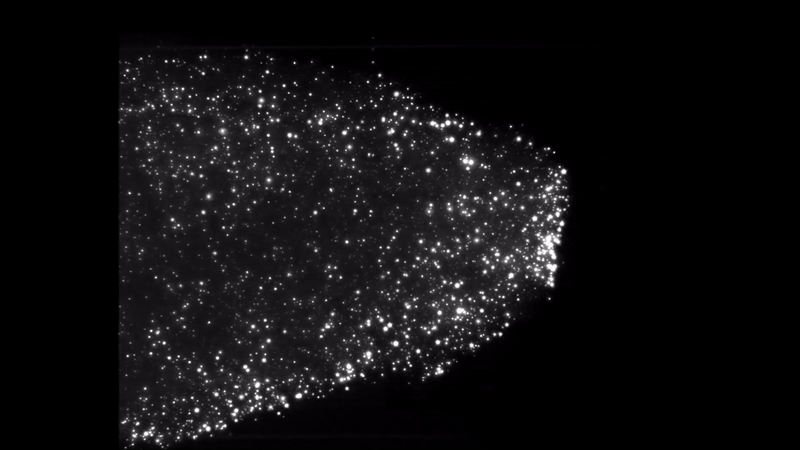
Discrete burning 2















 Germany
Germany
 Austria
Austria
 Belgium
Belgium
 Denmark
Denmark
 Spain
Spain
 Estonia
Estonia
 Finland
Finland
 France
France
 Greece
Greece
 Hungary
Hungary
 Ireland
Ireland
 Italy
Italy
 Luxembourg
Luxembourg
 Norway
Norway
 The Netherlands
The Netherlands
 Poland
Poland
 Portugal
Portugal
 Czechia
Czechia
 Romania
Romania
 United Kingdom
United Kingdom
 Slovenia
Slovenia
 Sweden
Sweden
 Switzerland
Switzerland

























