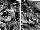
Figs. 1a/1b. Optical microscopy of haematoxylin-eosin stained cells fused after 6 min of µg and 1 day of subculturing (1a, left), and the identical 1g ground-based reference experiment (1b. right).

Others have shown that the cytoskeleton undergoes specific changes during the brief periods (6 min) of microgravity available in sounding rocket experiments. It is therefore possible that cell processes dependent on the cytoskeleton's structure and function are also influenced. A cell fusion experiment, initiated by a short electric pulse (electrofusion), was chosen as a model experiment for this sounding rocket experiment. Confluent monolayers of primary human skin fibroblasts, grown on coverslips, were mounted between two electrodes and fused by discharging a capacitor in a low- conductive medium. During a µg experiment in which nearly all the requirements for an optimal result were met (only recovery of the payload was delayed), the results indicated that 6 min of µg did not influence cell fusion: the percentage of fused products did not change. Within the limits of discrimination using fluorescence and electron microscopical methods, µg has no influence on the actin/cortical cytoskeleton just after electrofusion.
Experiments performed during long-duration µg with several kinds of cells have shown that important cellular functions such as cell division, T cell activation and metabolism are altered.1 The mechanisms involved in these changes are still unknown. The effect of gravity inside a cell seems to be very small due to the slight differences in density between the different organelles and the cytoplasm in comparison with the high thermal energy.2 Focusing of gravitational energy by the cytoskeleton seems to be a possible mechanism for gravity sensing by cells.3
Brief experiments aboard sounding rockets (6 min µg) have revealed that the cytoskeleton undergoes changes4 , so there is a possibility that cellular functions dependent on the cytoskeleton structure might also be a fected by short-term µg. Membrane fusion processes such as exocytosis, endocytosis, cell division and cell fusion, which seem to be dependent on the cytoskeleton structure,5 are therefore candidates for this type of µg experiment aboard a sounding rocket. As the timeline of the different types of membrane fusion processes determines whether an experiment can be flown in a µg period of several minutes, we have chosen cell fusion by electrofusion as a model system for the influence of µg on membrane fusion.
Experimental design
The experiment was designed to
account for the launch effect by fusing a group of 'control' cell
monolayers at 0 min µg (launch effect) and 6 min µg
(launch effect + µg effect). The fusion indices in these
µg experiments were compared with those of similar fusion
experiments simultaneously following the same timeline at the
ground site. The influence of µg plus electropulse on the
morphology and ultrastructure of the cells after fusion was
determined by fixing the cells shortly after applying the fusion
pulse. Later, the cells were processed further for optical,
fluorescence or electron microscopy.
Cells and media
Primary human skin
fibroblasts (local strain 81RD238) were cultured in Hepes
buffered medium M199 supplemented with antibiotics (100 IU/ml
penicillin; 0.1 mg/ml streptomycin; 0.05 mg/ml gentamycin) and
10% foetal calf serum. Two days before fusion, cells were seeded
on coverslips at 70% confluency (55 000 cells/cm²). The
fusion medium consisted of: Na2HPO45 mM;
KH2PO45 mM; MgCl2
1 mM; Sucrose 272 mM.
MASER 5/CIS-3 sounding rocket experiment
The electrofusion experiment was part of the CIS-3 module in the
MASER 5 campaign launched in April 1992 from Kiruna, Sweden. The
experimental schedule around launch and during µg is
described below. Three coverslips with human skin fibroblasts
were mounted 6 hr before launch between two electrodes (0.5 cm
separation) in the central compartment of each spring-loaded
Plunger Box Unit (PBU).6 The integration of the PBUs
into the CIS module took place 4 hr before launch. The
electropulse facility (EPF) was programmed to replace the culture
medium with fusion medium, to apply a single pulse of 250 V
(field strength 500 V/cm) by discharging a 68 mF capacitor for
10 ms to each PBU, and to change the fusion medium in the fusion
compartment back to either fixative or culture medium. All
experiments were performed at 37±0.2 C. The time schedule
was as follows:
-15 min µg: replacement of culture medium by fusion medium
-78 s µg: launch
0 min µg: application of electric pulse to first set of
PBUs (launch effect)
+½ min µg: fixing cytoskeleton experiment (launch effect);
replacement of fusion medium by culture medium
(launch effect)
+6 min µg: application of electric pulse to second set of
PBUs (launch + µg effect)
+6 min µg: fixation of cytoskeleton experiment (launch +µg effect);
replacement of fusion medium by culture medium
(launch + µg effect)
+8 min µg: end of µg period and re-entry of MASER 5
+3 hr re-entry: dismounting of PBUs and subculturing of fused cells
+27hr re-entry: fixation and staining of cultured fused cells
All experimental procedures used before and during the CIS-3 flight were tested beforehand. The 1 g ground experiments were performed in identical experimental environments using the same time schedule of experiments and recovery.
Determination of fusion percentage
After
dismantling the PBUs, the cells were cultured for 1 day. They
were then fixed in Bouin fixative and stained with haematoxyline-
eosine.7 Random cell fields on the coverslip (about
500 cells per coverslip) were examined and the number of nuclei
was counted in each microscopically observed cell.
FITC Phalloidin staining
To study the
actin cytoskeleton, cells grown on glass coverslips were first
fixed in the PBU in 2% buffered paraformaldehyde 30 s after
applying the fusion pulse. After dismantling the PBUs, the actin
cytoskeleton was subsequently stained using fluorescent
Phalloidin.8
Electron microscopy
To study the
ultrastructural morphology, cells grown on polystyrene coverslips
(Nunc) were first fixed in the PBU in 2% buffered
paraformaldehyde 30 s after applying the fusion pulse. After
opening the PBUs, the coverslips were stored in 4% buffered
paraformaldehyde 1% glutaraldehyde. Fixing (OsO4),
staining (tannic acid), embedding (epon) and further preparation
for electron microscopy was performed according to ref.9.
Electrofusion
To quantify cell fusion
(Figs. 1a/1b), we expressed the number of nuclei in polykaryons
(cells with more than one nucleus) as a percentage of the total
number of nuclei. A correction can be applied for the multi-
nucleated cells in a control culture without
electrofusion.10 When the data are arranged in such
a way (Table 1), the fusion index after 6 min of µg
(39.6±3.0%) is only slightly lower when compared with the
0 min µg launch control (43.1±3.1%). The fusion indices
of the ground controls, which were identical experiments in
principle as the cells were fused at the ground site at exactly
the same time as in CIS-3 during the µg period, differed
more (0 min: 38.3%; 6 min: 31.6%) than the µg and launch
control cultures.
Table 1. Effect of microgravity on the fusion index of human fibroblasts.
number of number of nuclei number of nuclei fusion
counted cells in monokaryons in polykaryons index
----------------------------------------------------------------------------------
0 min µg 3447 2576 2372 43.1% (40.0-46.2)
6 min µg 1675 1266 1013 39.6% (36.6-42.7)
0 min 1 g 1521 1145 939 38.3% (35.3-41.4)
6 min 1 g 1635 1291 805 31.6% (28.7-34.6)
----------------------------------------------------------------------------------
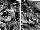
Figs. 1a/1b. Optical microscopy of haematoxylin-eosin stained
cells fused after 6 min of µg and 1 day of subculturing (1a, left),
and the identical 1g ground-based reference experiment (1b. right).
These differences reflect the spread in fusion indices of identical experiments in different PBUs. The µg experiments differed less than the 1 g ground controls. Therefore, the experiments under brief µg (0 min; 6 min) do not support a µg effect on the electrofusion process for attached cultured human fibroblasts.
Actin cytoskeleton
Cells were fused and
fixed at the beginning of a period of µg (two coverslips)
and in another series after 6 min of µg (two coverslips)
during sounding rocket flight (see 'Materials and methods' for
flight schedule). After fixing they were stained for actin with
fluorescent phalloidin in the laboratory, and compared with
ground-based reference experiments at 1 g. There was a weak
staining of the cell cytoskeletons that were subjected to fusion
and µg. No differences were apparent between the cells that
were fused immediately at the start of µg and those fused
at the end of µg. The cytoskeleton of the cells that were
fused at the ground site showed a more pronounced fluorescence.
So there seems to be a launch, but not µg, effect. As a
control, normal fibroblasts that were not subjected to fusion or
microgravity were stained together with the experimental samples.
The control cytoskeleton was much more distinct than that of the
experimental fusion mixtures from the PBUs. Thus the fusion
process itself also influences the cytoskeleton structure, as was
already known.11 It seems, therefore, that, within the
limits of discrimination, there was a launch effect on the
cytoskeleton structure after fusion. The following µg period
did not have an additional effect.
Cortical cytoskeleton The cells that were fused and fixed at the beginning and end of the µg period (for the flight schedule see 'Materials and methods') were also processed to observe the cortical cytoskeleton by electron microscopy. These cells were compared with 1 g reference experiments simultaneously fused and fixed at the ground site. After further fixing and embedding in Epon, the cells were cut perpendicular to and parallel with the plane of their coverslip. The ultrastructural morphology of the cortical cytoskeleton did not differ, within the limits of discrimination, between the different experiments (Figs. 2a/2b.). So launch and µg did not have any visible effect on the cortical cytoskeleton directly (30 s) after fusion.

Figs. 2a/2b. Ultrastructural morphology of cortical cytoskeleton
of skin fibroblasts fused and fixed after 6 min ofµg (2a, left),
and the identical 1 g ground-based reference experiment
(2b, right). The bars represent 0.5 µm.
We wish to thank Dr R. Huijser (Fokker Space), Mr H. Willemsen (Centrum voor Constructie en Mechatronica; CCM), Mr Aartman (National Aerospace Laboratory; NLR) and W. Boender (Centrale Instrumenten Dienst, Erasmus Universiteit; CID) for their support and cooperation. This project was financed by ESA (project number AO-SRLS/89/6-NL) and SRON (project number MG-029).



 SP1206
SP1206